If your patient continues to have trouble controlling their blood sugar, first evaluate whether the blood sugar and A1c goals are appropriate.
Physicians should individualize goals with the patient for those with medium risk for hypoglycemia using clinical judgment and patient agreement. Patients with end-stage or terminal co-morbid conditions should be maintained at glucose levels that prevent catabolism and symptoms, such as an A1C >8% which corresponds with an estimated average daily blood sugar of 183iv
| Goals for Patients at Low Risk for Hypoglycemia1 | |||
|---|---|---|---|
| Pre-Meal | Post-Meal | Bedtime | A1C |
| 70-130 | <180 | <180 | 6.5%-7.0% |
| Goals for Patients at High Risk for Hypoglycemiai | |||
|---|---|---|---|
| Pre-Meal | Post-Meal | Bedtime | A1Ciii |
| 100-180 | <250 | <250 | 7.0%-8.0% |
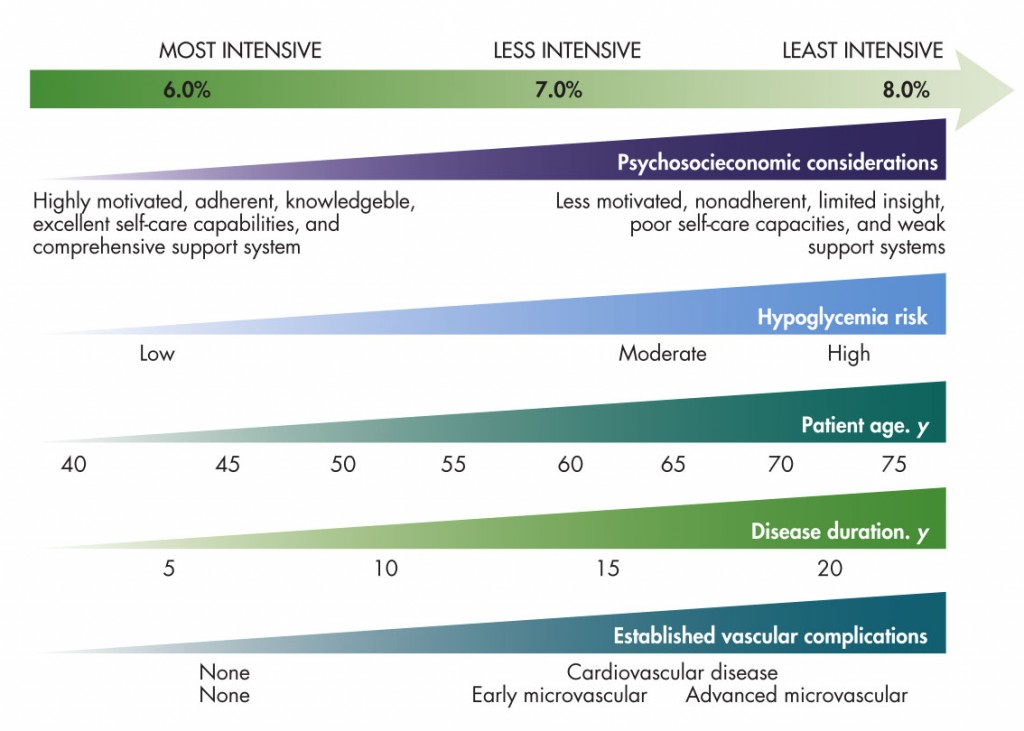
A number of factors should also be considered when setting an appropriate A1c goal for someone with type 2 diabetes. It should also be noted that A1c is not a perfect indicatorv as it does not reveal glycemic variability or other factors that may impact risk for complications. The graphvi below can help you to determine whether your patient’s goal is appropriate based on risks for hypoglycemic events, age, comorbidities, and other lifestyle factors. A more in-depth examination of recommended A1C targets based on clinical characteristics can be found in the following graph.
| Use the following checklist to determine if your patient may benefit from mealtime insulin. | ||
|---|---|---|
| 1. Is the patient’s A1C above their individualized goal? | Yes | No |
| 2. Is the patient’s basal insulin dose at or above 0.5 U/kg | Yes | No |
| 3. Is the patient’s fasting blood sugar at goal, but A1C still high? | Yes | No |
| 4. Is the patient’s post-meal blood sugar >180 for low risk patients or >250 for high risk? | Yes | No |
| The more yes responses, the more likely it is that your patient would benefit from mealtime insulin. However, before your make this transition, consider whether the basal insulin dose is adequate and whether there may be problems with adherence to the treatment plan. | ||
If your patient is still not able to control their blood sugars after you have determined that their A1C goal and their basal dose are appropriate, evaluate whether the patient is adhering to the treatment plan. Review the patient’s blood glucose logs if available to assess fasting and post-prandial blood sugars and patterns that may indicate non-adherence.
Also assess if there are any superimposed factors (added glucocorticoids, etc.). If there are issues in these areas, it is recommended that you address them before moving beyond basal insulin.
Once you determine that mealtime insulin is the appropriate approach, discuss with your patient whether basal/bolus therapy or pre-mixed insulin would be a better treatment option. Below are descriptions, along with the pros and cons, to help you and your patient think through what approach may be best.
| Pros | Cons |
|---|---|
| Background + rapid-acting insulin allows for greater flexibility. You can adjust your insulin intake to fit less regular schedules and to more/less carb intake. | Patients may need to take 2-5 injections each day depending on how many meals need mealtime insulin. Some people also split the long-acting insulin into morning and evening shots. |
| Studies have shown that adding just one mealtime dose of rapid acting insulin per day, given before the largest meal, improves glycemic control nearly as much as adding 2-3 doses per dayvii. | When rapid-acting insulin is added at dinnertime, this may result in lower bedtime blood glucose and this may necessitate a lower dose of background insulin. |
| It’s an easier transition for many patients because they are already on background insulin and they understand how insulin works. | There are two co-pays for basal/bolus insulin. One co-pay is for the background insulin and one co-pay is for the mealtime insulin. Patients will need to carry the insulin with them but the availability of pens has helped somewhat alleviate this concern. |
| Pros | Cons |
|---|---|
| Premixed insulin has only one co-pay and is best suited for a patient with a fairly predictable schedule with regular meals and with a lower risk of hypoglycemia. | Patients must eat regular meals or they will be at a greater risk for hypoglycemia. |
| Patients often need fewer shots (1-2 per day) than basal/bolus therapy. | Nocturnal hypoglycemia may be a greater concern with pre-mixed insulin. |
| Premixed R & N has the lowest cost. | Premixed Regular & NPH has a greater risk for hypoglycemia. |
| Premixed Humalog 50/50 is an option for patients who need larger doses of the rapid acting component because of high carbohydrate meals. | There is an increased need for between meal snacks. |
i Inzucchi S, Bergenstal R, Buse J. Management of Hyperglycemia in Type 2 Diabetes: A Patient-Centered Approach. Diabetes Care 2012.
ii Ibid.
iii Ibid.
ivHHN Fact Sheet
v Alarcon-Casas Wright, Hirsch. The Challenges of the Use of Glycemic Biomarkers in Diabetes: Reflecting on Hemoglobin A1C, 1, 5-Anhydroglucitol, and the Glycated Proteins Fructosamine and Glycated Albumin. Diabetes Spectrum 2012; 25:141-148.
vi Ismail-Beigi F et al. Ann Intern Med 2011;154:554-559
vii Davidson MB, Raskin P, Tanenberg, R, Vlajnic A, Hollander P. A Stepwise Approach to Insulin Therapy in Patients with Type 2 Diabetes Mellitus and Basal Insulin Treatment Failure. Endocrine Practice. 2011;17:3: 395-402.
1 Note: these goals are not evidence-based but are consensus opinion. Goals for any patient should be individualized further based on patient preference and clinical judgment.
2 Pre-meal goals for patients with a high-risk for hypoglycemia may vary and should be individualized.

Join our endocrine community and become a member! Only members receive access to a variety of member benefits that will enhance your career. If your membership has lapsed, rejoin today so that you can continue to receive your membership benefits.